• • •
"Mike and Jon, Jon and Mike—I've known them both for years, and, clearly, one of them is very funny. As for the other: truly one of the great hangers-on of our time."—Steve Bodow, head writer, The Daily Show
•
"Who can really judge what's funny? If humor is a subjective medium, then can there be something that is really and truly hilarious? Me. This book."—Daniel Handler, author, Adverbs, and personal representative of Lemony Snicket
•
"The good news: I thought Our Kampf was consistently hilarious. The bad news: I’m the guy who wrote Monkeybone."—Sam Hamm, screenwriter, Batman, Batman Returns, and Homecoming
April 25, 2011
A Piece Without So Much Math In It
By: Aaron Datesman
I realize that recent posts have had a lot of math in them, and are confusing. Unfortunately, this is unavoidable. The story is rather remarkable, and I'd like for it to be clear why you should believe it. This post, then, is a recap without so many mathy parts. Let's concentrate on the story instead.
The initial lesson, using radioactive Schwarzium, demonstrated that the half-life of an unstable nucleus represents a probability rather than a promise. When you have only four atoms of a radioactive substance, it's actually quite likely (6%) that all four of them will decay in one half-life. The decay process is statistical - and we all know how statistics never lie.
Next, as an aside, we took a moment to ask: how many atoms are there? We are generally given information about activity, in Curies or Becquerels or decays per second (those last two are the same, incidentally). The answer is, a reasonably low rate of 1 decay per second probably corresponds to hundreds of thousands or millions of atoms, all of which could decay at any time.
Then we learned the statistical rule which governs the decay of small numbers of unstable nuclei, and saw that it depends upon the average number of decays occurring in the sample. The rule gives the probability of encountering a number of decays N in the sample when the average number of decays one observes is another number, Nbar. It is physically possible to see 10,000 decays/sec when the average rate is only 5 decays/sec - although it's so unlikely that it's probably never happened in the entire history of the universe.
Finally, in the post before this one we made the commonsense observation that the decay of the unstable nucleus of a potassium atom in your big toe probably can't give you brain cancer. The insight here is that the decay of a nucleus affects only a limited volume around the site of the decay. (Well, for alpha decay, the volume is very small - around 0.0001 cubic millimeters. For beta decay, the volume is about 1 cubic millimeter; and for gamma decay, in many instances the interaction volume should rightly be the whole body.)
Therefore, if you're not a mathy guy or girl, the one piece of supporting information to take away from the previous post is that we should adjust the average rate of decay to reflect the proper size scale.
As an example, our bodies contain a background level of disintegrations of Potassium-40: about 40,000 every second. Potassium-40 is a beta emitter, so those 40,000 decays/sec should properly be spread out over a large number (around 70 million) of independent cubes 1mm on a side. This results in an average decay rate in each cube of around 0.001 decays/sec - which is a quite low rate.
Based upon that simple observation, we then applied the proper statistics, and found that very high decay rates (for short times) occur reasonably frequently even though the average decay rates are very low. Maybe this is surprising, but it's no different than flipping a coin one billion times and discovering that there was one series where it came up tails 74 times in a row.
It's a reasonable suspicion that a cell might not be capable of correctly repairing the damage from a large number of near-simultaneous decays in its vicinity. Therefore, this little oddity of statistical physics could have truly enormous health implications.
The scale of the concern is shown in the graph below.
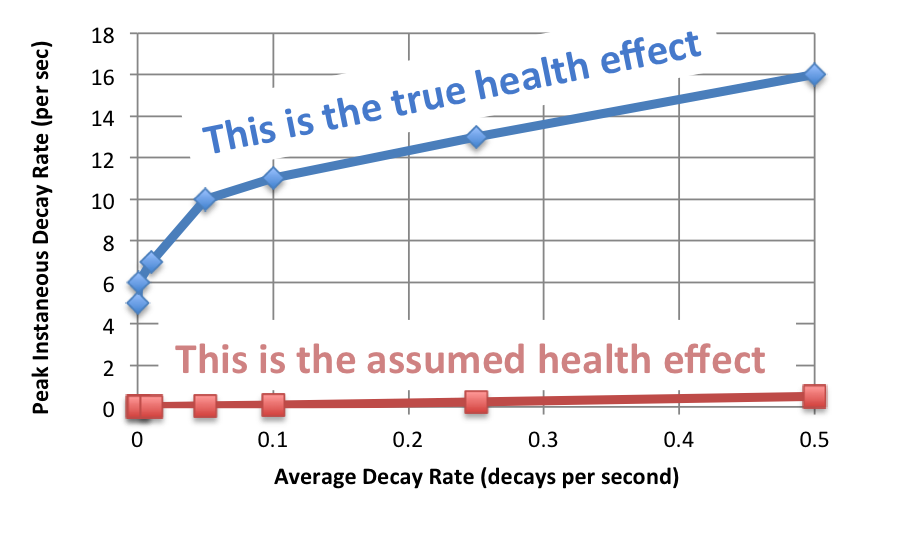
Sorry about all that math. (The numbers are different in this graph from those in the graph in the other post because I corrected an error and removed a factor accounting for the US population. There is no qualitative difference.)
I have a great deal more to say, but am leaving to go on a loooooong vacation which I very richly deserve. I have really appreciated the readers and comments; thanks!
— Aaron Datesman
Hypothetically speaking, say YOU had a health policy with Humana-Bedford and the big toe was misdiagnosted as a bunion, and matastasized into brain cancer?
Posted by: Mike Meyer at April 26, 2011 01:00 AMThanks for focusing attention on this important problem. I'd never thought of this problem before but now that I've read your posts I believe you're completely wrong. I don't expect you to reply to my comments, since so far you've made a point of ignoring them. But here we go:
1. Your gaussian tail estimate is wrong.
2. You told us that "if more than two atoms of Schwarzium decay in your vicinity, you will suffer acute radiation sickness and die." In other words, what matters is the magic threshold of decays above which you die. In your example you seem so proud of, if you double the rate of 10^-3 to 2.10^-3 you pass 10 one thousand times more often. So in your body 1000 volume units will get the lethal dose with good probability instead of 1. But, according to you, that doesn't matter. What matters is that the peak will go from 10 to a measly 12. Yeah, sure.
3. After telling us average decays were at least 1 now you inform us they're tiny. Nice! The upside is that your last 3 posts can be summarized in one sentence: "the Poisson standard deviation is the square root of the mean, hence the dominant term when less than 1, hence a concave function of the mean for a fixed deviation." That explains everything you tried to say with such difficulty. The only problem is that it's irrelevant. Why? Because you told us so! You told us what matters is the prob of crossing a threshold somewhere in your body. And that is widely superlinear. If anything, the linear model is overly pessimistic.
4. I'll ask one more time: what are the green objects in your previous chart? What kind of scientist displays a graph without explaining what's plotted. It's baffling.
I give up.
Happy vacation.
Nice article, thanks for the information.
Posted by: sewa mobil at April 26, 2011 02:39 AMPerhaps WE should look at the point where the amount of decay ionizes enough DNA molecular groups to split the ladder. Isn't that what industrial wastes and other carcinogens do, ionize DNA molecular groups?
Posted by: Mike Meyer at April 26, 2011 04:05 AMEnjoy that long vacation, Aaron. (I hope you're not going to Japan.)
Posted by: N E at April 26, 2011 06:17 AMbobs - I don't mean to ignore anybody's questions. When I started writing these posts, my wife was away in a foreign country on business. She is back now, thankfully, so I have less free time. Sorry about that. I'm a regular guy with a life and a job, and I write these posts in my spare time late at night.
1) Why is the Gaussian tail estimate wrong? What should the answer be? (I assume you mean 2x10^-9 for 42000 per second against an average of 40000.) The answer could very well be wrong. I did the calculation quickly and didn't think very hard about it.
2)The example with Schwarzium is just a game to illustrate that half-life is a probability, not a promise. I didn't mean it to extend directly to the more serious stuff.
About the second half of this point, I'm not clear what you mean exactly. I will try to clarify what I meant to say a little bit.
This post really refers to a model. We take a human body and break it into 70 million interaction volumes, because in reality a single decay can't directly injure cells outside of this small volume. Then we ask, what rates of decay do we expect to encounter in this volume?
It's just a model. Is it sensible? You are welcome to form your own opinion. The only benchmark for its usefulness is whether it agrees with observation.
Now, this is what the model says: for the stated rates of average decays, most interaction volumes will experience around one dose equal to the plotted data (between around 10-30) in one year. In my opinion, it's hardly crazy to think that the cancer incidence would track with the size of that dose.
You are welcome to form your own opinion, of course.
3) The average decay rate is benchmarked to a volume. I could take the limit where that volume contains, on average, only one unstable nucleus. In that case, the average decay rate over all the volumes would be nearly zero. Then, when there is one decay, I suppose I could say "OMG that's a 1 ZILLION PERCENT INCREASE CANCER!@!!$". And that would be dumb.
The point is that there's a biologically-appropriate interaction volume. (There's also a biologically-appropriate time interval, which may not be one second. I have ignored this complication so far.)
I think you're applying the example of the Schwarzium atoms to this post, where I did not mean it to apply. I apologize for the confusion. It occurred to me when I wrote the 4-coins example, but I decided to use it anyway 'cuz I thought it was funny.
4) The kind of scientist who displays charts without explaining them is a) the kind who doesn't get paid, and b) the kind who provides links the readers could follow to figure stuff out for their own damn selves.
The answer to your question, though - I think - is that Mangano plotted excess over the expected rate. So 0.4, at the bottom of the vertical axis, is 40% over the expected rate of lung cancer, 1 is double the expected rate, and 2 (if it were on the graph) would be triple.
If you want my attention and are willing to be polite, you can e-mail me. I also am figuring this stuff out on my own.
Posted by: Aaron Datesman at April 26, 2011 10:56 AMAaron, have a great loooooong vacation. This post helped me better understand the previous ones, so thanks for taking the time to put it up before taking off. Bon voyage!
Posted by: N at April 26, 2011 11:57 AMThank you, Dr. Datesman!
Posted by: Amandasaurus at April 26, 2011 12:56 PMAlso, I like that when your wife is out you fill your time with physics. A man after my own heart, indeed.
Posted by: Amandasaurus at April 26, 2011 12:58 PMThis is a post from a friend of mine on Facebook today:
"Today is the 25th anniversary of the Chernobyl Explosion that devastated several countries, including Belarus. 23% of the Belarus is still contaminated and it will be hundreds of years before that area is declared clear. I was 4. I remember asking my Mom if the radiation will be over soon and her telling me that yes, it will. I am healthy, thanks to various programs that took us away from the contaminated areas sometimes for 10 months out of the year, but so many people, including my Mom, are very sick because of that. There is nothing anybody can do. Today, I remember those who risked and lost their lives trying to save the world and my thoughts go to those who are trying to save the world now in Japan. I hope and pray that a disaster like this never happens again."
...again, I just want to say thank you for taking the time to pursue this topic.
Posted by: Amandasaurus at April 26, 2011 02:32 PMWe don't "need a weatherman to know which way the wind blows" but we do need a little math now and then.
Posted by: Dredd at April 26, 2011 02:44 PMWhen the time from treatment to decess in incurable cancer patients is plotted on semilog paper the result is a straight line that does not intersect at point 1 but above it on the ordinate. Between point one and the beginning of the straight line a curve develops a curve whose amplitude varies with the severity of the disease. Earlier cases show a straight segment displaced considerably, most advanced cases show no incurvation at all. The exponential that describes the curve requires the use of the extrapolation number which is the point at which the straight segment of the curve when drawn upwards intersects the ordinate. This effect is seen in cell cultures and the theory was that it represents a number of targets that have to be hit before the exponential process develops in its simplicity.
I make notice of this because the curves that you display though not plotted on semilog paper have a strong resemblance to those in the cancer process.
Aaron:
1. 2.10^-9 is indeed wrong. Not even close. (Remember you're 10 sigmas away!)
2. when the mean of a poisson is much less than one then the number of sigmas tells you where you are for a given probability (you can forget the mean). But the standard deviation of a poisson is the square root of the mean, hence the concavity you've been looking for. There's nothing more to it.
3. In the previous post, you said it was "at least one unit of vol getting the bad dose." Now it's become "most units of vol." Which is which? Earlier you said it's a threshold that matters, not the actual size: 10 you die and 12 you... still die. Now you say it ain't so. Which is which? My point was that when the rate goes up the damage is much greater. Think of it this way. With 1 dollar you can shoot one 1-inch bullet into someone's body and with 2 dollars you can shoot one 1.2-inch bullet. Seems like not a big improvement. But the point is that with 2 dollars you also get, free of charge, 1000 extra 1-inch bullets! So when you drop below the threshold the effect is highly superlinear.
4. You're wrong about your chart: 1 is the null value. And you still haven't said a word about the squares and the triangles. Why 2 different shapes? This is really my main criticism. You teach science by intimidation, not even bothering to define what you're talking about. If you put up a chart, then please explain what it means. The excuse you have no time is hard to take. Don't write half a dozen posts then: write only 1 and explain what you're doing. Peace.
@bobs - Oh, yeah. I missed that one by sixteen orders of magnitude. Thanks.
Posted by: Aaron Datesman at April 26, 2011 11:10 PMGreatings,
Perdonen, es limpiado
http://www.elcoru.com/
SuperSonic



